Our fuel cell testing systems meet all your requirements. Test your fuel cells according to your specific requirements, e.g., under the following conditions:
- Direct connection of the air supply to the fuel cell
- Non-preconditioned process air supply to the test chamber
- Treated process air supply to the test chamber and temperature-controlled hydrogen
We also ensure any necessary explosion protection. To this end, we equip our fuel cell testing systems with the appropriate safety devices:
- H2 sensors in the process air piping or in the test chamber
- Indirect heat transfer heating in the process air conditioning
- Indirect heat transfer heating in the test chamber
- Pressure relief valves/bursting discs in the test chamber ceiling
- Purge device with compressed air/fresh air
- Temperature-controlled
Performance parameters *
-55°C to +200°C
Type of construction *
The following device types are available:
The reaction
in the
fuel cell
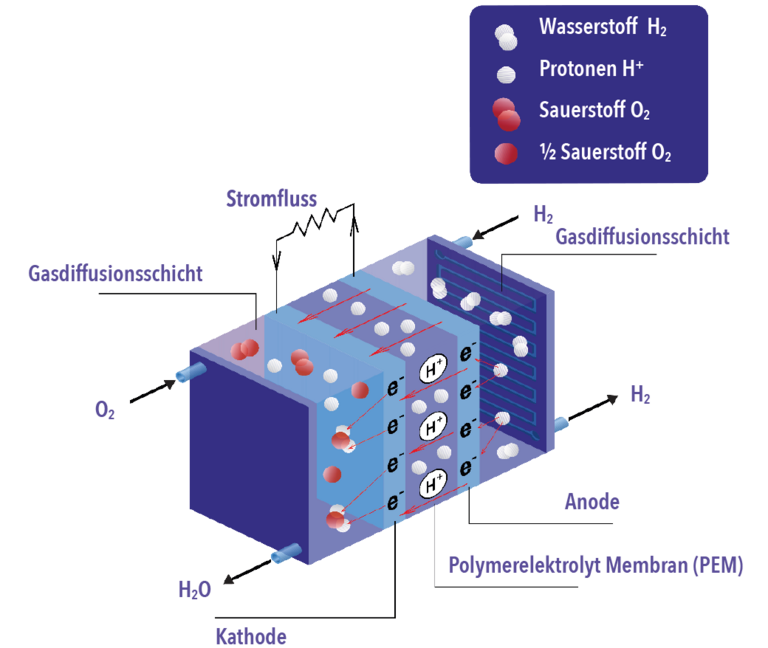
The heart of the fuel cell consists of two electrodes: the anode and the cathode. They are separated from each other by a membrane. This membrane is impermeable to gases. Each of the electrodes is coated with a catalyst, for example nickel or platinum.
This is how the cell works, using the example of a polymer electrolyte membrane fuel cell:
Hydrogen (H2) is fed to the anode and oxygen (O2) to the cathode. On the anode side, the hydrogen is oxidized to protons, releasing electrons. The electrons flow from the anode to the cathode via an external circuit. Electricity flows. The protons diffuse through the electrolyte to the cathode. Here, the protons and electrons combine with the supplied oxygen to form water (H2O). A fuel cell thus continuously produces electrical energy, heat energy, and water.
With our fuel cell testing systems, you can precisely test the functionality and quality of your cells. We would be happy to advise you!